- Wed. Apr 24th, 2024
Latest Post
Student Publications Leaders Recognized for Multimedia Content and Sales Expertise at National Awards Ceremony
During an awards ceremony on April 19, three WKU Student Publications student leaders were recognized by College Media Business & Advertising Managers. Emma Bayens, a senior photojournalism major from Louisville,…
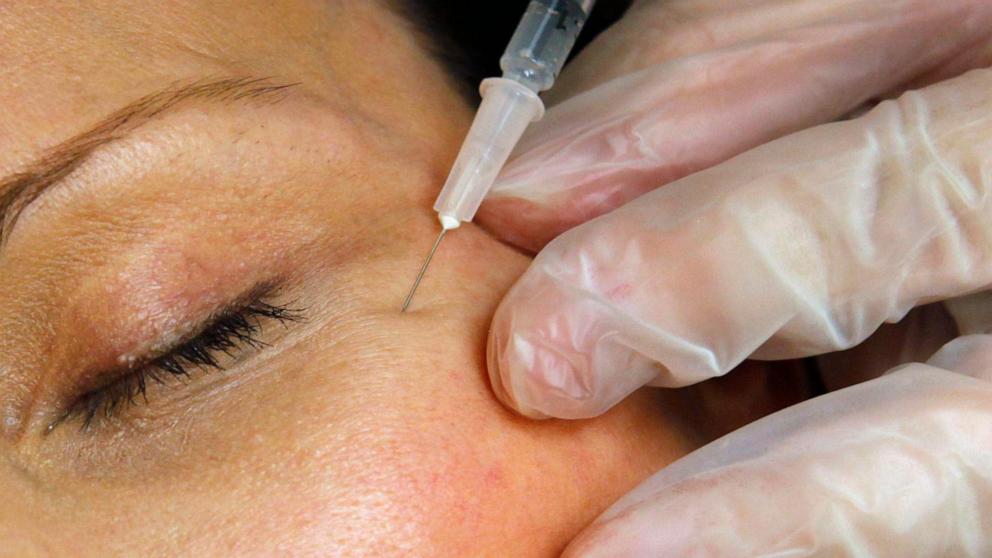
Unlicensed Practitioners Causing Serious Health Issues with Counterfeit Botox Injections: What You Need to Know
In early November, more than 20 individuals in 11 states were affected by counterfeit Botox injections. These injections were administered by unlicensed or untrained individuals in settings such as homes…
Kate Gutman’s Appointment as Ziff Davis’ Executive Vice President and General Manager of Technology Media Group Marks New Chapter in Company’s Growth Strategy
Kate Gutman, a seasoned digital media executive with a proven track record in leading editorial organizations and driving diversified revenue growth, has been appointed as the Executive Vice President and…
Donum Estate: Leading the Way in Sustainable Viticulture with Green Business Award
Donum Estate has been recognized as the recipient of this year’s Green Business Award by the Sonoma Valley Chamber of Commerce and City of Sonoma. This honor was bestowed after…
Unveiling China’s Espionage Operations in Western Countries: The Ethics and Privacy Dilemmas of Ghostwriting and Academic Integrity.
Beijing’s extensive intelligence operations in Western countries are being highlighted by the recent arrest of an assistant to German MEP Maximilian Krah, who is a main candidate for the far-right…
Revolutionizing Defense Maintenance: CTMA Partners Meeting Showcases Innovative Solutions for Improved Returns on Investment
Looking to gain insight into how the Department of Defense (DOD) can be modernized for better returns on investment in maintenance and sustainment (M&S) initiatives, technology innovators are invited to…
Tesla Unveils Revamped Model 3 Performance Sedan, Amid Mixed Q1 Earnings and Sales Decline
Tesla unveiled a revamped version of its Model 3 Performance sedan on Tuesday, boasting improved performance, reduced energy consumption, and enhanced handling capabilities. The new model starts at $52,990 and…
Ethical Guidelines for Artificial Intelligence: A Significant Step towards Responsible Development and Growth
The Council of Ministers has approved a bill on artificial intelligence that aims to bring order and regulation to this rapidly evolving sector. The document includes measures such as imprisonment…
António Guterres Warns Humans are Damaging Earth’s Ecosystems and Urges Swift, Fair Action from G20 Leaders
On International Planet Day, António Guterres, the Secretary-General of the United Nations, issued a warning about the global chaos caused by pollution, extinction of species, ecosystems, and greenhouse gases. He…
Breaking News: Young Woman Rescued from Kidnapper’s Grip; Affiliate Marketing on the Rise for Girl Model Videos and BongaCash
On Sunday morning, a young woman was able to reach the phone and call her mother for help after being kidnapped and held captive by a suspect. With the assistance…